1996
Osteogenics Biomedical enters the dental bone grafting market with a dense PTFE membrane. The company is incorporated with a mission of developing dental bone grafting products to increase the predictability of regenerative procedures.

Shane Shuttlesworth was named president in October 2007. Prior to this position, Shane was the director of marketing & operations. Prior to assuming that position, he served several management positions in regulatory affairs, quality assurance and production. His current responsibilities include overseeing operations, marketing/sales, international business development, quality assurance and regulatory affairs. He originally joined Osteogenics Biomedical in 2001 as production manager.
A native of Lubbock, TX, Shane graduated summa cum laude with a bachelor of science degree in biology from Lubbock Christian University in 2001, received an MBA in general business and an advanced certificate in health organization management from Texas Tech University in 2003, and received a masters of science in regulatory affairs and services from St. Cloud State University in 2015.
Shane currently serves on Lubbock Christian University’s Young Alumni Advisory Board. Outside interests include basketball, football, fly fishing, and spending time with his daughter.
Liz Bastian joined Osteogenics in May 2021 as Director of Regulatory Affairs. In this role, she oversees all aspects and operations of the regulatory department.
Liz was born and raised in India. She moved to United Arab Emirates (UAE) in 2009 and then to Toronto, Canada in 2011. Several years later, she moved to Chicago, IL for her previous job, and she just recently moved to Lubbock, TX, where Osteogenics’ headquarters is located.
Prior to joining Osteogenics, Liz has worked for several medical device manufacturers and pharmaceutical companies as a regulatory affairs professional. She holds a bachelors and a masters degree in biotechnology and obtained her PMP certification in 2016.
Liz enjoys cooking, spending time with her husband and son, and traveling.
Blake Baucum was named director of sales in February 2022 after serving as the associate director of sales for five years and a regenerative product specialist for five years prior to that. His current responsibilities include overseeing sales activities, training the sales and marketing staff on the technical aspects of Osteogenics’ products, and supporting the marketing and regulatory teams in various projects.
Blake was born and raised in Lubbock, TX, and graduated from Texas Tech University in 2009 with a bachelor of science degree in biology. While attending Texas Tech, and for a short time after graduating, Blake worked at a local orthodontist’s office, filling various roles within the practice. He then took a position selling capital equipment for a larger dental supply company.
Blake cherishes spending time with his wife, two sons, and his extended family. He is an avid golfer, enjoys traveling, and loves spending time in the outdoors fly fishing or camping.
Courtney Riggan was named director of engineering in July 2016 after serving as senior engineering manager for two and a half years and quality engineer for three years prior to that. Her current responsibilities include overseeing design & development, risk management, complaint management, and some regulatory submissions.
Born and raised in Lubbock, TX, Courtney graduated from Texas A&M University in 2008 with a bachelor of science degree in biomedical engineering and received an M.S. in mechanical engineering from Texas Tech University in 2010.
Courtney enjoys traveling, playing Frisbee golf, and spending time with her husband and two children.
Russ Rowan returned to Osteogenics as director of marketing in January 2024. He previously served as Ostoegenics’ director of marketing from 2012 – 2022 and spent a short time working in another industry during most of 2023. Throughout his career at Osteogenics, Russ has been an integral part of the company’s sales and marketing activities. Russ has had the opportunity to work on projects with various teams across different departments inside and outside the commercial part of the business.
Russ was born and raised in Lubbock, TX, and graduated from Texas Tech University in 2002 with an undergraduate degree in finance. He also received an executive MBA from Texas Tech in 2016.
Russ enjoys spending time with his family and getting a round of golf in whenever time allows.
Salem O’Steen was named Director of Quality Assurance in December 2023 after serving as the Associate Director of Quality Assurance for seven years and as a quality manager for nine years prior to that. His current responsibilities include overseeing all quality management system processes such as CAPA, document control, internal and external audits, management review, finished product release, and continuous quality system improvements. He originally joined Osteogenics Biomedical in 2005 as a production associate.
Salem is native to Lubbock, TX, and graduated with a bachelor of science degree in biology from Lubbock Christian University in 2005. Salem obtained a certification from American Association of Tissue Banks as a Certified Tissue Bank Specialist (CTBS) in 2009; he has a Six Sigma Green Belt certification; and he received a masters of science in medical technology in quality from St. Cloud State University in 2017.
Salem's outside interests include cooking, exercising, camping, and spending time with his wife and two kids.
Evan Cain was named Director of Research and Development in 2023. Prior to this position, Evan was the Associate Director of Research and Development since 2018. Evan originally began his career with Osteogenics in 2012 as a quality manager. His current responsibilities include overseeing the research and development of new products and product line improvement. Evan works closely with quality, regulatory, and operations teams to bring new products to market.
A native of Plains, TX, Evan graduated magna cum laude with a bachelor of science degree in chemistry from Texas Tech University in 2010. He then went on to receive a masters of science degree in biotechnology from Texas Tech University in 2013.
In his free time, Evan enjoys bass fishing, golfing, and spending time with his wife and two kids.
Anibal Manzo joined Osteogenics in November 2023 as Director of Chanel Sales, where he is responsible for overseeing, supporting, and developing Osteogenics channel partners and global distribution network. Before joining Osteogenics, Anibal held several sales and marketing leadership roles at various U.S.-based dental companies. Most recently, he served as the VP of Sales and Marketing for the dental division at NovaBone Products, a position he held for three years.
Anibal was born in Caracas, Venezuela, and graduated from the Universidad Simon Bolivar in 1996 with a degree in International Business. He also earned a B.S. in Business from the Universidad Simon Rodriguez in 2000. Anibal moved to the United States in 2001, continuing his career in marketing and internal business within the dental implant industry.
Anibal values spending time with his wife and daughter. He enjoys outdoor activities, cooking, exercising, cycling, and traveling.
Headquartered in Lubbock, Texas, Osteogenics Biomedical is a leader in the development of innovative dental bone grafting products serving periodontists, oral & maxillofacial surgeons, and clinicians involved in regenerative and implant dentistry throughout the world. Osteogenics offers a complete line of bone grafting products including: enCore® combination and mineralized allografts, Zcore™ porcine xenograft, Cytoplast™ PTFE membranes, Cytoplast™ RTM collagen membranes, Vitala™ porcine pericardium collagen membranes, Zmatrix™ porcine peritoneum collagen membranes, Cytoplast™ PTFE suture, NovaBone® synthetic putty, the Pro-fix™ Precision Fixation System, and Resorba® dental sutures.
We pride ourselves in having the best customer service in the industry. Whether it’s placing an order or inquiring about a technical issue, you will always be greeted by a friendly voice who will work to quickly address your needs. Please note that online orders are for U.S. and Canadian customers only. For international orders, please contact us directly or visit our Global Network page to find a distributor.
Osteogenics Biomedical enters the dental bone grafting market with a dense PTFE membrane. The company is incorporated with a mission of developing dental bone grafting products to increase the predictability of regenerative procedures.

Osteogenics introduces a new patented surface technology - the Regentex™ textured surface - to the dense PTFE membrane. This 3rd generation PTFE membrane combines the benefits of a membrane occlusive to bacteria with a textured surface that helps stabilize the membrane and soft tissue flap.
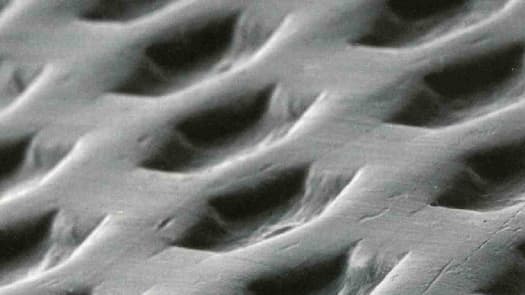
Osteogenics introduces the first nitride-coated ridge augmentation mesh - Osteo-Mesh™ TM-300.

Osteogenics acquires Permaridge® synthetic ridge augmentation matrix from CeraMed Dental. Permaridge® helps correct alveolar ridge resorption, providing denture wearers with more stable and comfortable dentures.

Osteogenics relocates to a larger manufacturing facility in Lubbock, TX.

Osteogenics receives a technique patent for treating alveolar bone defects using dense PTFE membranes - the Cytoplast Technique™. The Cytoplast Technique™ for socket grafting is the first technique utilizing membranes left exposed, thereby reducing surgical time, preserving soft tissue architecture and increasing keratinzed mucosa. Because the Cytoplast™ dense PTFE membranes are impervious to bacteria, they are especially well-suited to be left exposed in the oral environment.
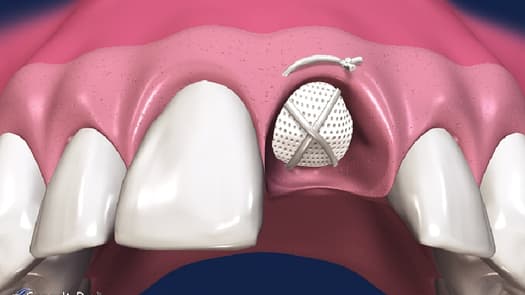
Osteogenics expands its regenerative portfolio with the addition of Cytoplast™ PTFE Suture and Cytoplast™ RTM collagen membranes. The company is one of only two companies in the world to manufacture PTFE suture.

Osteogenics receives ISO 9001 and ISO 13485 quality certifications, demonstrating compliance with strict European quality guidelines.

Osteogenics moves its corporate headquarters and manufacturing operations into a new 7,000 square-foot state-of-the-art facility in Lubbock, TX.

The company receives MDD/93/42/EEC certification, allowing the company to expand into Europe by distributing under the CE mark.

The company applies its Regentex™ surfaced technology to its line of titanium-reinforced membranes. Osteogenics also introduces four new titanium-reinforced application-specific membrane sizes.

Osteogenics Clinical Education™, a division of Osteogenics Biomedical, is established with a mission of providing interactive, hands-on clinical education in bone grafting and implant dentistry.

Osteogenics introduces two application-specific titanium-reinforced membrane sizes. The new sizes are the first to incorporate pilot holes and a broader titanium frame.
170 clinicians from six countries and three continents attend Osteogenics Clinical Education's™ first global bone grafting symposium in Scottsdale, Arizona.

Osteogenics signs an agreement with META Advanced Medical Technology in Italy, giving the company distribution rights in the U.S. and Canada for its dental bone grafting products, including its autogenous bone collectors Micross and Safescraper®, and crestal sinus lift kit, SinCrest.
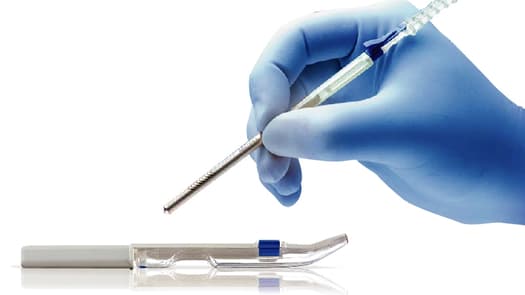
Osteogenics introduces the Pro-fix™ Precision Fixation System. Pro-fix™ is first introduced with self-drilling membrane fixation screws and will soon include self-drilling tenting screws and self-tapping bone fixation screws.

Osteogenics introduces enCore® Combination Allograft and enCore® Mineralized Allograft, the first dental bone grafting product introduced by Osteogenics. The combination allograft is the first particulate dental bone grafting product to combine mineralized and demineralized bone in a single bottle.

Osteogenics moves into a new 10,000 square-foot manufacturing facility, adding to its corporate headquarters campus in Lubbock, Texas.

Osteogenics undergoes major renovations to its corporate headquarters to maximize space for company growth.

Osteogenics acquires exclusive distribution rights to sell the synthetic, osteostimulative NovaBone® Dental products in the U.S. and Canada. Within the NovaBone® product line is an especially unique product, NovaBone® Dental Putty, a pre-mixed moldable putty that is available in a variety of delivery systems.
Osteogenics introduces Zcore™ porcine xenograft particulate, an osteoconductive anorganic bone mineral derived from porcine cancellous bone. Hyper-porosity of the porcine cancellous matrix and intra-particle space reduces bulk density of the graft, allowing 88 – 95% empty space for bone growth.

Osteogenics acquires the exclusive North American distribution rights for the premium German dental suture brand, Resorba®. Resorba® sutures are made from unique materials that are designed especially for dentistry and are available with black 300-series stainless steel needles that enhance visualization in the mouth.

Osteogenics introduces the newest product to complement the family of enCore® allografts - enCore® OD allograft, comprised of 30% mineralized cortical bone and 70% mineralized cancellous bone. enCore® OD is designed to be used with osseodensification (OD) protocols.

Osteogenics expands its regenerative portfolio with the addition of absorbable collagen wound dressings - Cytoplast™ RTMPlug, RTMFoam, and RTMTape.

Osteogenics launches its newest collagen membrane, Zmatrix, a natural/native non-cross-linked porcine peritoneum collagen membrane that is pliable enough to contour to its environment but substantial enough to not adhere to itself.

RPM™ Reinforced PTFE Mesh is introduced. Its unique circular macroporous design allows for direct contact between the bone graft and periosteum, allowing
naturally occurring revascularization and infiltration of cells into the bone graft. RPM's titanium frame maintains space essential for horizontal and vertical ridge augmentation, and the PTFE mesh easily conforms to tissue contours.

Osteogenics acquires distribution rights for the Swann-Morton® premium micro-serrated blades. These blades are designed with a unique cutting edge that results in a consistently sharp blade. The edge design combines a micro-serrated edge with a razor edge that provides the user with a tactile sensitivity improving depth control, while also creating equal, smooth tissue margins.

We are looking for the best and brightest minds to join our team. Browse our list of current job and internship opportunities, and electronically submit your resume using the form provided.
Find a Distributor
Subscribe to Our Newsletter
Be the first to know about:







